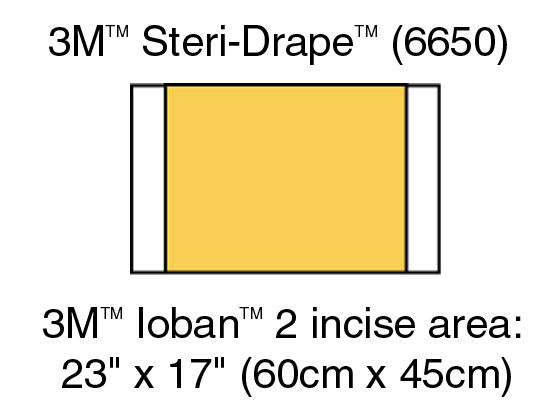
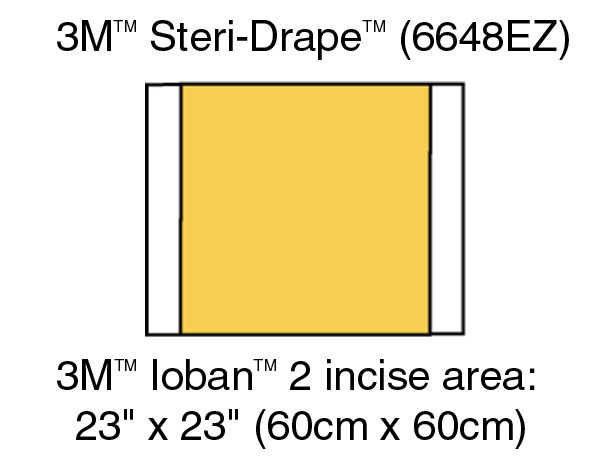
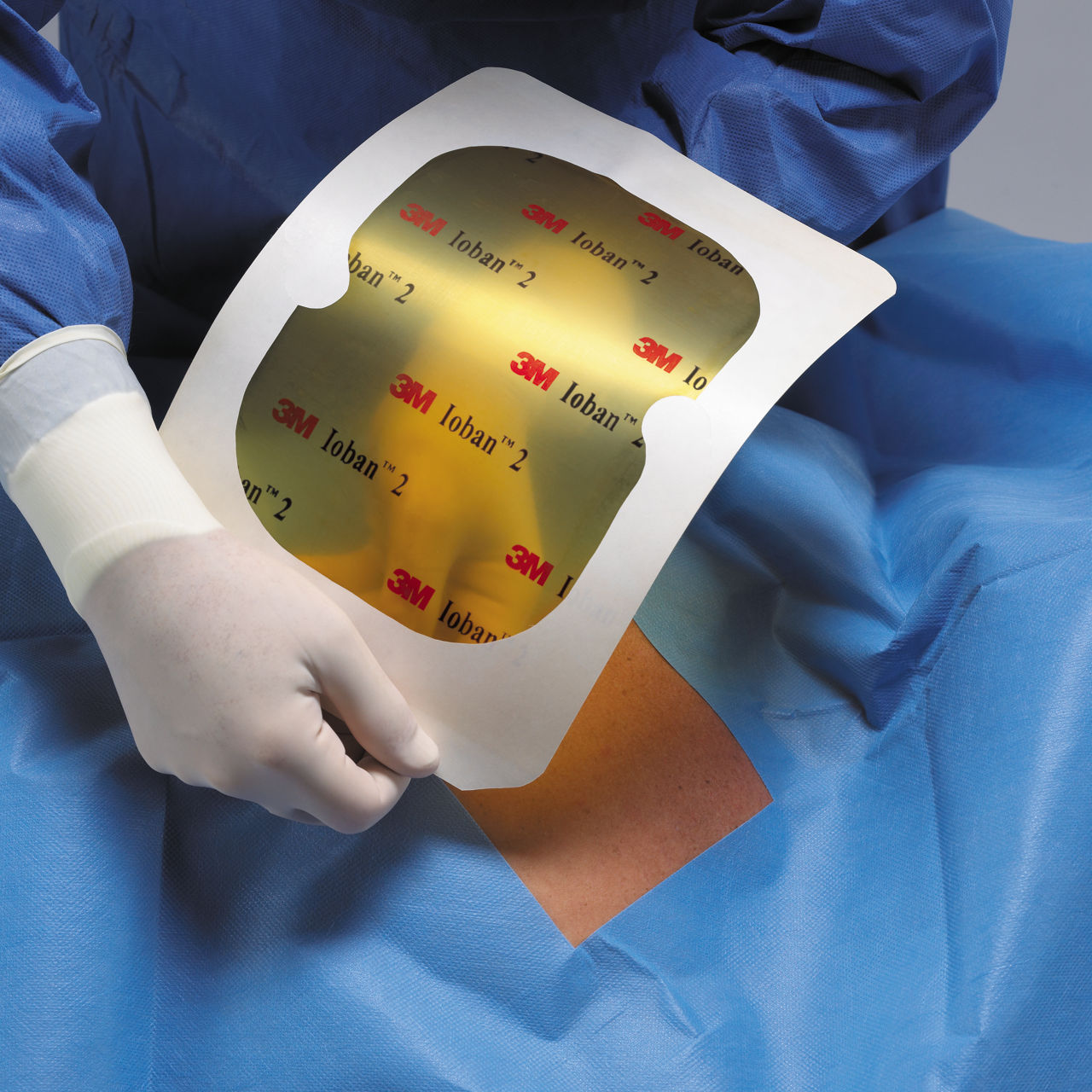
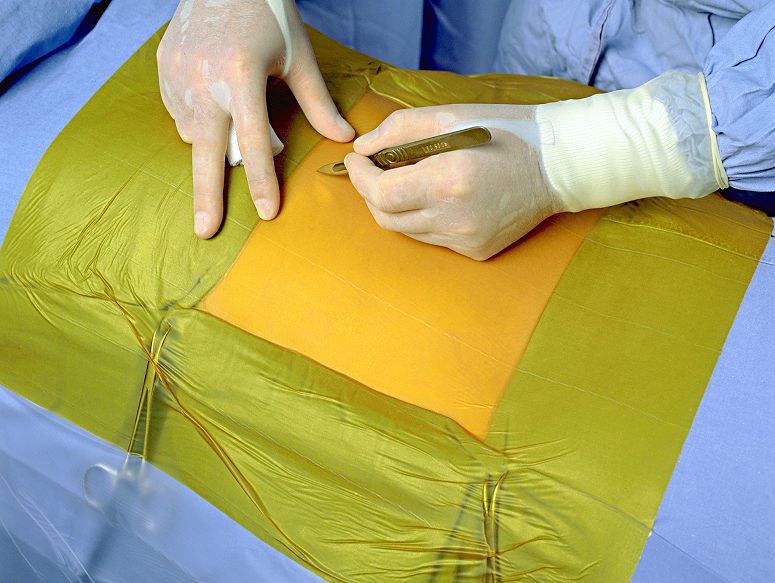
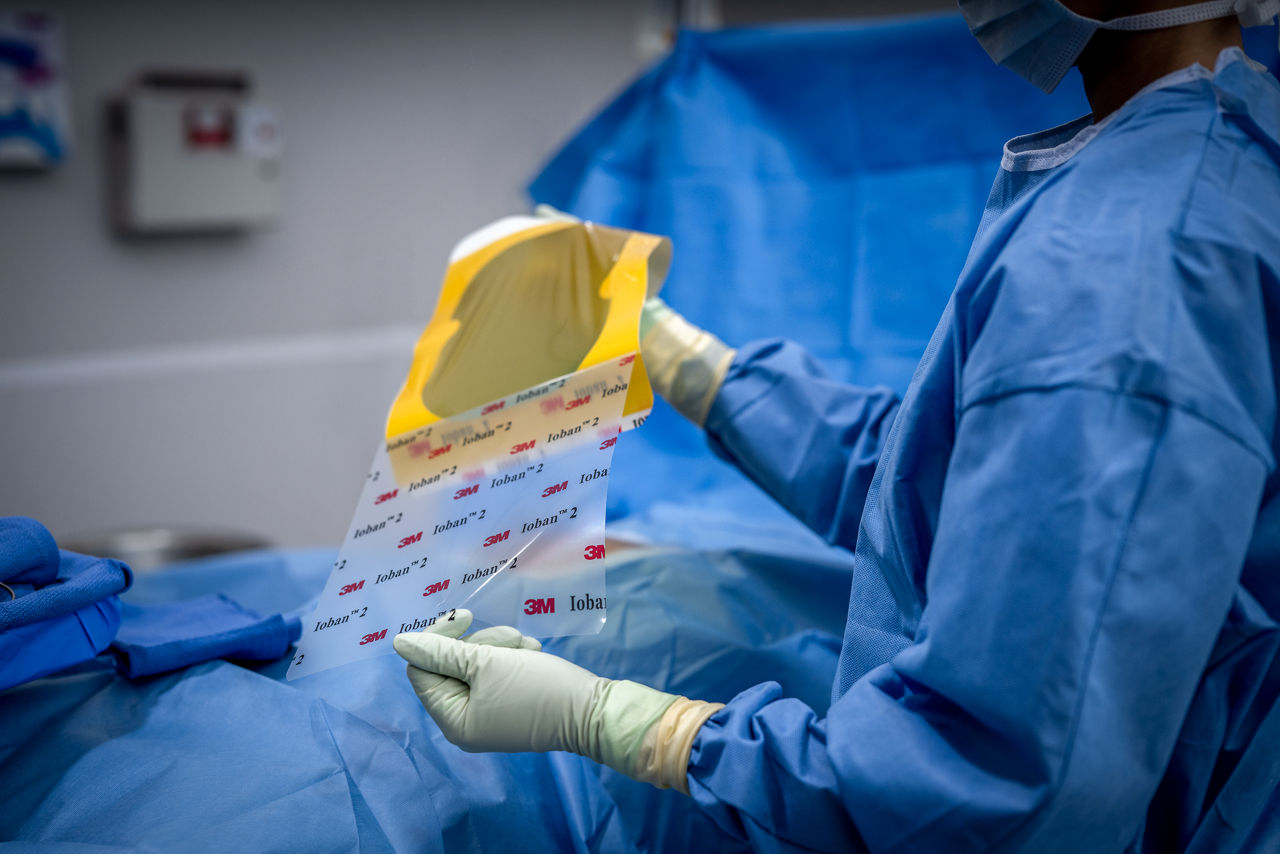
6650EU IOBAN ANTIMICROBIAL INCISE DRAPE
About the product
3M™ Ioban™ 2 Antimicrobial Incise Drapes can help reduce the risk of surgical site infections by immobilising bacteria and providing continuous antimicrobial activity.
Product details
- Continuous antimicrobial activity: Ioban 2 Antimicrobial Incise Drape provides continuous broad-spectrum antimicrobial activity to help reduce the risk of surgical site contamination.
- Complies with NICE guidance on the use of incise drapes. Ioban is an iodophor impregnated drape with clinical evidence to support its performance.
- Immobilises bacteria: Ioban 2 Antimicrobial Incise Drape immobilises bacteria on the skin, helping to prevent migration into the surgical incision area.
- Provides broad spectrum antimicrobial activity
- High moisture-vapour transmission rate (MVTR) for breathability ensuring adhesion throughout the procedure
- Low memory stretch allows limb mobilisation or heavy retraction with reduced tension to the skin
- Latex-free for patient and healthcare worker safety
- Polyliner for easy and fast application
Suggested Applications
- Incise draping with continuous antimicrobial activity for surgical procedures
Legal
¹ Hesselvig AB, Arpi M, Madsen F, Bjarnsholt T, et al; ICON Study Group. Does an Antimicrobial Incision Drape Prevent Intraoperative Contamination? A Randomized Controlled Trial of 1187 Patients. Clin Orthop Relat Res. 2020;478(5):1007-1015. ² Rezapoor M, Tan TL, Maltenfort MG, Parvizi J: Incise Draping Reduces the Rate of Contamination of the Surgical Site During Hip Surgery: A Prospective, Randomized Trial. J Arthroplasty 2018, 33:1891-5. ³ Bejko J, Tarzia V, Carrozzini M, et al. Comparison of Efcacy and Cost of Iodine Impregnated Drape vs. Standard Drape in Cardiac Surgery: Study in 5100 Patients. J Cardiovasc Transl Res 2015, 8:431-7. ⁴ Sworn K , Poku E, Thokala P, et al. Efectiveness of iodine-impregnated incise drapes for preventing surgical site infection in patients with clean or clean contaminated wounds: A systematic literature review and cost-consequence analysis. J Perioper Pract 2023:17504589221139603.Product specifications
| Brand |
Brand
Ioban™ |
| Overall Length (Imperial) |
Overall Length (Imperial)
25.98 in |
| Overall Width (Metric) |
Overall Width (Metric)
45 cm |
| Overall Length (Metric) |
Overall Length (Metric)
66 cm |
| Category Name |
Category Name
Surgical Drapes |
| Material |
Material
Plastic |
| Overall Width (Imperial) |
Overall Width (Imperial)
17.72 in |
There was an error processing your request. Please try again later.